Part II Publications in peer-reviewed journals
2020-present
Abstract: Using stereochemical insights and synthetic chemistry principles, we successfully achieved the total synthesis of two diastereomers of thermolide D, as well as the proposed structure of thermolide E. By comparing their NMR spectra and demonstrating coelution of the synthetic and natural products on a semipreparative HPLC column, we confirmed the correct structures of thermolides D and E. Additionally, the stereochemical approach used in this study provides a reliable framework for future structural determinations of fungal-derived polyketides.
Abstract: The absolute configuration of cubensic acid was predicted using the “Biochemistry-based Rule” and confirmed through total synthesis. Key steps in the synthesis included a titanium-mediated aldol reaction, a Paterson anti-aldol reaction, and a vinylogous Mukaiyama aldol reaction.
Abstract: Alternapyrone, a bioactive polyketide produced by the fungal host Aspergillus oryzae, is biosynthesized by a polyketide synthase encoded by the alt1-5 gene cluster. Despite its known bioactivity, the stereochemical configuration of the three stereogenic centers in its polyketide backbone has remained unresolved. In this study, we determined the complete stereostructure of alternapyrone using an integrative approach that combines predictive, rule-based stereochemical analysis with experimental validation through total synthesis. The efficient total synthesis enabled the precise assignment of the hypothesized stereochemistry by matching the synthetic product to the natural compound. This comprehensive study conclusively established the absolute configuration of alternapyrone.
Abstract: A second-generation formal synthesis of psymberin was successfully developed, incorporating several notable advancements. Central to this approach is a challenging Heck reaction, which effectively unites a sterically demanding aryl moiety with a terminal alkene, showcasing the method’s robustness under these conditions. Additionally, the construction of the isocoumarin scaffold was accomplished through a Pd-mediated cyclization, underscoring the efficiency and precision of this key transformation. Notably, this improved route to De Brabander’s advanced intermediate from the 2,6-trans-tetrahydropyran fragment demonstrates significant advantages over the previous synthesis. The revised method requires seven fewer steps, reducing complexity and enhancing practicality. Moreover, the overall yield has been markedly improved, increasing from 5.9% in the earlier route to 9.3%, reflecting a considerable gain in efficiency. These optimizations highlight the method’s potential for broader applicability in the synthesis of psymberin and related compounds.
Abstract: The structural groups of 2-oxindole and tricyclic 3a-hydroxy-hexahydropyrrolo-[2,3-b]indole (HO-HPI) are important pharmacophores. Chemical synthesis of complex alkaloids containing a 2-oxindole or HO-HPI moiety, especially the latter one, has been a long-standing challenge. Herein, we characterized the P450 enzyme AfnD, and its homologue proteins, HmtT, ClpD, KtzM, and LtzR, as cyclopeptide 2-oxindole and HO-HPI monooxygenases (cpOPMOs) that could introduce a 2-oxindole or HO-HPI moiety into the tryptophan-containing cyclopeptides in a pH-dependent manner. A universal catalytic mechanism was proposed for the five cpOPMOs, in which two conserved residues, Asp and Ser (Thr for LtzR), were proposed to divergently open the epoxide intermediates, thereby forming a 2-oxindole or HO-HPI moiety. Based on this, we constructed ten Asp or Ser/Thr mutants of cpOPMOs, which could synthesize cyclopeptides with an HO-HPI or 2-oxindole structure, selectively, under appropriate reaction conditions. All of the ten cpOPMO mutants exhibited high substrate promiscuities and usually performed well with cyclopeptides that are structurally similar to their native substrates. Overall, our work discovers a group of intriguing P450 enzymes, the cpOPMOs, and provides a powerful enzymatic toolkit for the selective synthesis of HO-HPI- or 2-oxindole-containing cyclopeptides.
Abstract: Roselipin 1A, a bioactive natural glycolipid isolated from marine fungal metabolites, presents an unresolved configuration of its nine stereogenic centers within the polyketide chain. Herein, we elucidate the comprehensive stereostructure of roselipin 1A through an integrative approach combining predictive rule-guided analysis with synthetic chemistry. The efficient total synthesis facilitated the unequivocal confirmation of the hypothesized stereochemistry for roselipin 1A, thereby establishing its precise molecular configuration.

Abstract: The absolute configurations of the fungal-derived reduced polyketide eucalactam B were initially predicted using the “Biochemistry-based rule” and later confirmed through its first successful total synthesis. This accomplishment involved key reactions such as Brown crotylation, the Paterson anti-aldol reaction, cross-metathesis, and macrolactamization, furnishing eucalactam B in 19 linear steps, with an overall yield of 6.0%. The high degree of alignment between the synthetic compound and the natural product provided strong validation for the accuracy of the “Biochemistry-based rule” in predicting the absolute configurations of fungal-derived reduced polyketides.
Abstract: Actin stabilizers that are capable of interfering with actin cytoskeleton dynamics play an important role in chemical biology. Rhizopodin, a novel actin stabilizer, affects the actin cytoskeleton at nanomolar concentrations and exhibits potent antiproliferative activities against a range of tumor cell lines with IC50 values in the low nanomolar range. Herein, we report the total synthesis of rhizopodin based on a late-stage oxazole ring formation strategy, whose success demonstrates the feasibility of late-stage oxazole ring formation in the synthesis of complex oxazole containing natural products. Other features of the synthesis include a Nagao aldol reaction, a Suzuki coupling, a Yamaguchi esterification, a modified Robinson–Gabriel synthesis of the oxazoles, and a bidirectional Ba(OH)2-mediated Horner–Wadsworth–Emmons (HWE) reaction.
Abstract: The synthesis of the tetrahydrofuran/α-hydroxyl lactone fragment of mandelalide B has been achieved in a concise and highly stereoselective manner. The key steps in our synthesis are a mild Horner-Wadsworth-Emmons olefination to create the key carboxylate containing olefin, and a highly stereospecific iodine induced cyclization to construct the α-hydroxyl lactone moiety.
Abstract: The absolute stereochemical configurations of acremolides A and B were predicted by a biochemistry-based rule and unambiguously confirmed through their total syntheses. The features of the total syntheses include sequential Krische’s Ir-catalyzed crotylation, Brown’s borane-mediated crotylation, Mitsunobu esterification reaction, and cross-metathesis reaction. The efficient total synthesis enabled clear validation of the predicted stereochemistry for acremolides A and B.
Abstract: Harnessing the photoredox-mediated decarboxylative 1,4-addition reaction and the unusual use of silver carbonate to promote N-acylation, the first total synthesis of incarnatapeptins A and B has been achieved, which unambiguously confirmed the structure of these natural products.
Abstract: Natural products with diverse functional groups and stereogenic centers have inspired therapeutics and underpin the modern drug discovery process. Their three-dimensional molecular structures need to be unambiguously determined in order to be realized as clinical candidates or to achieve further activity-guided structural optimization. Although recent advances in spectroscopic methods have made it possible for researchers to determine the structures of microgram samples of complex natural products, there is no universally accepted method for determining the relative and absolute configuration of a naturally occurring compound. We report the determination of the full stereostructure of valactamide A, an eight-stereogenic-center-containing fungal metabolite by the synergy of prediction rule-guided analysis and chemical synthesis. The expedient total synthesis resulted in unambiguous verification of the predicted stereochemistry for valactamide A.
Abstract: Total synthesis of the proposed noursamycin A has been accomplished, which disproves the original structural assignments. The synthetic strategy described herein has also been employed in the first total synthesis of nicrophorusamide A, a cyclopeptide that is structurally related to noursamycin A.
Abstract: The first total syntheses of cyclic depsipeptides colletopeptide A and colletotrichamide A, have been accomplished. The key advanced intermediate, a cyclic tridepsipeptide derivative, was constructed using a sequence of transformations that features asymmetric Brown crotylation, cross metathesis, Yamaguchi esterification, ozonolysis, and macrolactamization. A late-stage incorporation of the mannose fragment completed the synthesis of colletotrichamide A, and the desilylation of the common intermediate gave rise to colletopeptide A, which led to unambiguous confirmation of the absolute stereochemistry of the aforementioned natural products.
Abstract: A novel hydrogen bond surrogate-based (HBS) α-helix mimetic was designed by the combination of covalent H-bond replacement and the use of an ether linkage to substitute an amide bond within a short peptide sequence. The new helix template could be placed in position other than the N-terminus of a short peptide, and the CD studies demonstrate that the template adopts stable conformations in aqueous buffer at exceptionally high temperatures.
Abstract: The first asymmetric total synthesis and validation of the structural assignment of des-thiomethyllooekeyolide A (3) is described, which features a Shiina macrolactonization and a late-stage pyran–hemiketal formation. The eight stereogenic centers of the C16-polyketide chain were installed by sequential aldol and crotylation reactions.
Abstract: Aurantinins (ARTs) are a group of antibacterial polyketides featuring a 6/7/8/5-fused tetracyclic ring system and a triene alkyl chain with a carboxylic terminus. Previous studies assigned their planar structures and proposed ART B as 17-O-glycosylated ART A with a rare 3-keto sugar. In this work, the stereochemical structures of ARTs A and B were assigned by combining the spectroscopic and computational methods with biosynthetic studies. Biosynthetic investigations aided in the assignment of ART A’s absolute configuration and provided critical evidence for determining the configuration of ART B’s 3-ketosugar moiety, demonstrating that appropriate biosynthetic investigations can be very useful in resolving the complicated structures of certain natural products.
Abstract: The stereocontrolled total synthesis of the antitumor natural product thioamycolamide A has been accomplished in 14 longest linear steps and an overall yield of 19.1%. The central feature of our convergent route to this family of novel macrocyclic natural products is the preparation of the β-alkylthio amide subunit through an auxiliary-controlled, diastereoselective sulfa-Michael addition.
Abstract: Mycotoxin cyclochlorotine (1) and structurally related astins are cyclic pentapeptides containing unique nonproteinogenic amino acids, such as β-phenylalanine, l–allo-threonine, and 3,4-dichloroproline. Herein, we report the biosynthetic pathway for 1, which involves intriguing tailoring processes mediated by DUF3328 proteins, including stereo- and regiospecific chlorination and hydroxylation and intramolecular O,N-transacylation. Our findings demonstrate that DUF3328 proteins, which are known to be involved in oxidative cyclization of fungal ribosomal peptides, have much higher functional diversity than previously expected.
Abstract: The asymmetric total synthesis of four diastereomers of laingolide A was achieved, which led to the unambiguous assignment of the stereochemistry of the natural product. The salient features of the convergent, fully stereocontrolled approach were a copper-catalysed stereospecific Kumada-type coupling, a Julia-Kocienski olefination and an RCM/alkene migration sequence to access the desired macrocyclic enamide.
Abstract: The first total synthesis of the thiazole-containing cyclic depsipeptide pagoamide A, is detailed. The longest linear sequence of the liquid-phase synthesis comprises 9 long linear steps from simple known starting materials, which led to the unambiguous structural confirmation of pagoamide A.
Abstract: Highly reducing polyketide synthases (HR-PKSs) produce structurally diverse polyketides (PKs). The PK diversity is constructed by a variety of factors, including the beta-keto processing, chain length, methylation pattern, and relative and absolute configurations of the substituents. We examined the stereochemical course of the PK processing for the synthesis of polyhydroxy PKs such as phialotides, phomenoic acid, and ACR-toxin. Heterologous expression of a HR-PKS gene, a trans-acting enoylreductase gene, and a truncated non-ribosomal peptide synthetase gene resulted in the formation of a linear PK with multiple stereogenic centers. The absolute configurations of the stereogenic centers were determined by chemical degradation followed by comparison of the degradation products with synthetic standards. A stereochemical rule was proposed to explain the absolute configurations of other reduced PKs and highlights an error in the absolute configurations of a reported structure. The present work demonstrates that focused functional analysis of functionally related HR-PKSs leads to a better understanding of the stereochemical course.
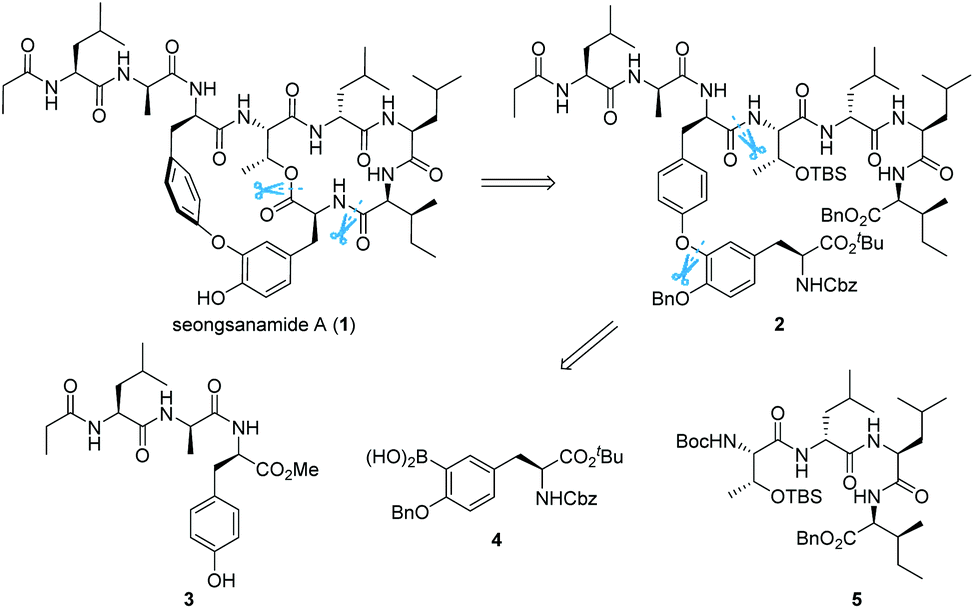
Abstract: The first total synthesis of antiallergic depsipeptide seongsanamide A has been achieved and also confirmed the relative and absolute stereochemistry of the natural product. Highlights of the convergent route include the use of Miyuara borylation, Chan-Evans-Lam coupling for the effective assembly of isodityrosine subunit and the identification of an effective macrocyclization site in very high conversion. The longest linear sequence leading to seongsanamide A was 12 steps, with an overall yield of 12.7%.
“Nine-Step Total Synthesis and Biological Evaluation of Rhizonin A” Qingchao Liu, Langlang Liu, Ranjala Ratnayake, Hendrik Luesch, Yian Guo, Tao Ye Chinese Journal of Chemistry 2020, 38, 1280-4.
Abstract: We have achieved the total synthesis of an architecturally and biologically intriguing cyclic polypeptide, rhizonin A (1), in an exceptionally concise and convergent fashion. The strategic route entails 9 longest linear steps to elaborate commercially available materials into the natural product with an overall yield of 9.7%. The brevity of sequence and high overall yield was fueled by the judicious selection of chemical tactics. Rhizonin A (1) showed weak inhibitory effects on the cell viability of HCT116 colorectal cancer cells and this activity was dependent on hypoxia-inducible factors.
“Asymmetric Total Syntheses of Kopsane Alkaloids via a PtCl2-Catalyzed Intramolecular [3+2] Cycloaddition” Xuelei Jia, Honghui Lei, Feipeng Han, Tao Zhang, Ying Chen, Zhengshuang Xu, Pratanphorn Nakliang, Sun Choi, Yian Guo, Tao Ye. Angew. Chem. Int. Ed. 2020, 59, 12832-6
Abstract: A concise and asymmetric total synthesis of five kopsane alkaloids which share a unique heptacyclic caged ring system was accomplished. The key transformation in our sequence involved a remarkable PtCl2 catalyzed intramolecular [3+2] cycloaddition, which allowed for the rapid assembly of pentacyclic carbon skeletons bearing 2,3-quaternary functionalized indoline. Expeditious construction of diverse indoline scaffolds with excellent control of diastereoselectivity demonstrated the broad scope and versatility of this key transformation
“Total Synthesis and Biological Evaluation of Kakeromamide A and Its Analogues” Meng Zhao, Yi Xiao, Satoshi Otsuka, Yoichi Nakao, Yian Guo1, Tao Ye Frontiers in Chemistry 2020, 8, doi: 10.3389/fchem.2020.00410
Abstract: Kakeromamide A (1), the first marine cyclopeptide inducing neural stem cells differentiation into astrocytes, was synthesized in 12 longest linear steps and 14% overall yield. Using this synthetic approach, four analogues of kakeromamide A were prepared and evaluated for neural differentiation- modulating activity.
“Proteomic study reveals the involvement of energy metabolism in the fast antidepressant effect of (2R, 6R)-hydroxy norketamine” Shafiq Ur Rahman, Qiang Hao, Kaiwu He, Yumeng Li, Xifei Yang, Tao Ye, Tahir Ali, Qiang Zhou, Shupeng Li, Proteomics – Clinical Applications, 2020, https://doi.org/10.1002/prca.201900094
Abstract: Depression is a major disabling psychiatric disorder, causes severe financial burden and social consequences worldwide. Recently, (2R, 6R)-hydroxynorketamine (HNK), a metabolite of ketamine, showed strong antidepressant effects through an N-methyl-D aspartate (NMDA) antagonizing independent mechanism. In the current study we tried to identify the potential intracellular molecules and pathways that might be involved in different therapeutic effects underlying HNK as compared to NMDA antagonist MK-801.
“Total Synthesis of Dysoxylactam A” Mingze Yang, Wenquan Peng, Yian Guo, Tao Ye Lett. 2020, 22, 1776-9
Abstract: The total synthesis of a potent multi-drug-resistant reverser, dysoxylacatam A (1), was achieved in a highly efficient and stereocontrolled fashion. The highlights of the strategy enlisted an iterative combination of lithiation−borylation tactics including Aggarwal homologation and Matteson homologation, Brown crotylation, Krische allylation, and ring-closing metathesis to forge the macrocycle.
“Reductase of Mutanobactin Synthetase Triggers Sequential C−C Macrocyclization, C−S Bond Formation, and C−C Bond Cleavage” Min Wang, Zhoujie Xie, Shoubin Tang, Ee Ling Chang, Yue Tang, Zhengyan Guo, Yinglu Cui, Bian Wu, Tao Ye, Yihua Chen Lett. 2020, 22, 960-4
Abstract: Mutanobactins (MUBs) and their congeners that contain a macrocycle and/or a thiazepane ring are lipopeptides from Streptococcus mutans, a major causative agent of dental caries. Here we show that the C-terminal reductase domain of MubD releases the lipohexapeptide intermediates in an aldehyde form, which enables a spontaneous C−C macrocyclization. In the presence of a thiol group, the macrocyclized MUBs can further undergo spontaneous C−S bond formation and C−C bond cleavage to generate diverse MUB congeners.