Publications
127.Diversity-Oriented Synthesis of Natural Products via Gold-Catalyzed Cascade Reactions
Yueqing Gu, Ceheng Tan, Jianxian Gong*, Zhen Yang*
Synlett 2018, 29(12): 1552-1571
This account describes our group’s latest research in the field of diversity-oriented synthesis of natural products via gold-catalyzed cascade reactions. We present two general strategies based on gold-catalyzed cycloisomerization: a gold-catalyzed cascade reaction of 1,7-diynes and a pinacol-terminated gold-catalyzed cascade reaction. We highlight our development of synthetic methods for the construction of biologically active natural products by using these two strategies.
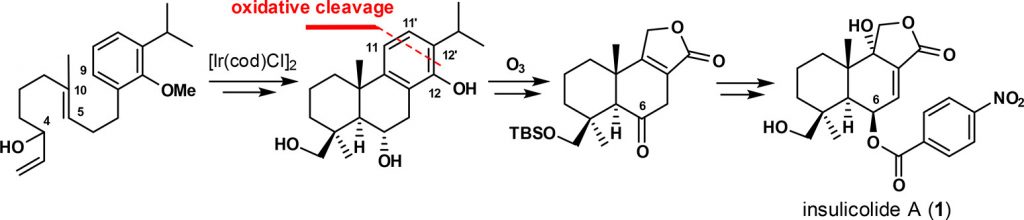
126.Asymmetric Total Syntheses of Insulicolide A, 14-O-Acetylinsulicolide A, 6β,9α-Dihydroxy-14-p-nitrobenzoylcinnamolide, and 7α,14-Dihydroxy-6β-p-nitrobenzoylconfertifolin
Yang Lai, Nan Zhang, Yi Zhang, Jia-Hua Chen*, and Zhen Yang*
Org. Lett., 2018, 20 (14), 4298–4301
Asymmetric total syntheses of insulicolide A, 14-O-acetylinsulicolide A, 6β,9α-dihydroxy-14-p-nitrobenzoyl cinnamolide, and 7α,14-dihydroxy-6β-p-nitrobenzoylconfertifolin have been achieved for the first time. The key steps in the synthesis include: (1) an iridium-catalyzed enantioselective polyene cyclization to construct the drimane core bearing two all-carbon quaternary chiral centers at C4 and C10 and (2) a cascade ozonolysis of the phenol ring to form the lactone fragment of the target molecules.
125.Asymmetric Total Synthesis of Lancifodilactone G Acetate. 1. Diastereoselective Synthesis of CDEFGH Ring System
Tian-Wen Sun, Dong-Dong Liu, Kuang-Yu Wang, Bing-Qi Tong, Jia-Xin Xie, Yan-Long Jiang, Yong Li, Bo Zhang, Yi-Fan Liu, Yuan-Xian Wang, Jia-Jun Zhang, Jia-Hua Chen*, Zhen Yang*
J. Org. Chem., 2018, 83 (13), 6893–6906
The stereoselective construction of the CDEFGH ring system of lancifodilactone G is described. The key steps in this synthesis are (i) ring-closing metathesis for formation of the oxa-bridged eight-membered ring; (ii) an intramolecular Pauson–Khand reaction for construction of the sterically congested F ring; and (iii) sequential cross-metathesis, hydrogenation, and lactonization reactions for installation of the anomerically stabilized bis-spiro ketal fragment of lancifodilactone G.
124.Asymmetric Total Synthesis of Lancifodilactone G Acetate. 2. Final Phase and Completion of the Total Synthesis
Kuang-Yu Wang, Dong-Dong Liu, Tian-Wen Sun, Yong Lu, Su-Lei Zhang, Yuan-He Li, Yi-Xin Han, Hao-Yuan Liu, Cheng Peng, Qin-Yang Wang, Jia-Hua Chen*, Zhen Yang*
J. Org. Chem.2018.83 (13), 6907–6923
The asymmetric total synthesis of lancifodilactone G acetate was accomplished in 28 steps. The key steps in this synthesis include (i) an asymmetric Diels–Alder reaction for formation of the scaffold of the BC ring; (ii) an intramolecular ring-closing metathesis reaction for the formation of the trisubstituted cyclooctene using a Hoveyda–Grubbs II catalyst; (iii) an intramolecular Pauson–Khand reaction for construction of the sterically congested F ring; (iv) sequential cross-metathesis, hydrogenation, and lactonization reactions for installation of the anomerically stabilized bis-spiro ketal fragment of lancifodilactone G; and (v) a Dieckmann-type condensation reaction for installation of the A ring. The strategy and chemistry developed for the total synthesis will be useful in the synthesis of other natural products and complex molecules.
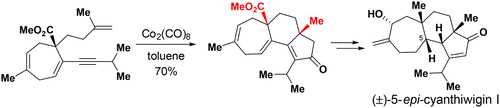
123.Stereoselective Total Synthesis of (±)-5-epi-Cyanthiwigin I via an Intramolecular Pauson–Khand Reaction as the Key Step
Yuanyuan Chang, Linlin Shi, Jun Huang, Lili Shi, Zichun Zhang, Hong-Dong Hao*, Jianxian Gong*, and Zhen Yang*
A convenient approach to the construction of the 5–6–7 tricarbocyclic fused core structure of cyanthiwigins via a Co-mediated Pauson–Khand reaction as a key step has been developed. The cyathane core intermediate obtained by this strategy was used in the concise synthesis of (±)-5-epi-cyanthiwigin I. The developed chemistry paves the way for the total synthesis of structurally diverse cyanthiwigins.