Publications
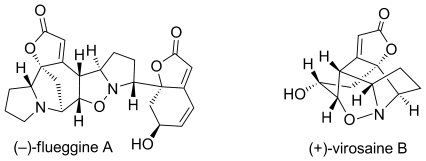
72.Stereoselective Total Syntheses of (−)-Flueggine A and (+)-Virosaine B
Dr. Hao Wei, Chuang Qiao, Gang Liu, Prof. Dr. Zhen Yang, Prof. Dr. Chuang-chuang Li
Angew. Chem. Int. Ed. 2013, 52, 2, 620
• Chosen as “Hot Paper” by the Editors.
• Top 3 Most Accessed Article in 11/2012.
• Highlighted in PKUSZ NEWS, 2012.
• Highlighted in Chemistry World, January, 2013.
• Highlighted in Synfacts, 2013, 9, 237 (“Synfact of the Month”).
Convergent approach: The total syntheses of (−)-flueggine A and (+)-virosaine B (see scheme) have been accomplished in a concise and convergent manner. Key steps in these approaches were relay ring-closing metathesis reactions for rapid construction of the key intermediates, and 1,3-dipolar cycloaddition reactions for the formation of the natural products.
71.Asymmetric Total Syntheses of Ansamacrolactams (+)-Q-1047H-A‑A and (+)-Q-1047H-R‑A
Shouliang Yang, Yumeng Xi, Rong Zhu, Lin Wang, Jiahua Chen, and Zhen Yang
The total syntheses of ansamacrolactams (+)-Q-1047H-A-A (16) and (+)-Q-1047H-R-A (17) have been achieved for the first time in 17 steps, leading to the reassignment of the relative stereochemistries and absolute configurations of their natural counterparts. The key steps in the synthetic work included an asymmetric chelation-controlled vinylogous Mukaiyama aldol reaction for the stereoselective synthesis of the syn-aldol adduct 7b and an intramolecular SmI2-mediated Reformatsky reaction for the formation of the macrocyclic lactam 14.

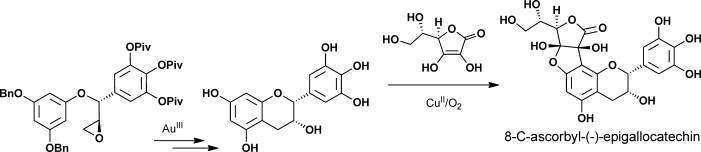
70.Enantioselective Total Syntheses of (+)-Gallocatechin, (−)-Epigallocatechin, and 8-C-Ascorbyl-(−)-epigallocatechin
Dr. Guang Lin, Dr. Le Chang, Dr. Yongxiang Liu, Dr. Zheng Xiang, Prof. Dr. Jiahua Chen,*, Prof. Dr. Zhen Yang*
Reading the tea leaves: The enatioselective total syntheses of 8-C-ascorbyl-(−)-epigallocatechin was accomplished by CuII-mediated oxidative coupling of ascorbic acid and (−)-epigallocatechin as a key step. Also, the asymmetric total syntheses of tea-leaf extracts (+)-gallocatechin and (−)-epigallocatechin were achieved by Au-catalyzed intramolecular cycliarylation of the precursor epoxide and Sharpless dihydroxylation.
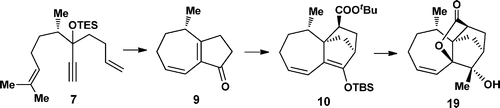
69.Synthesizing the Tetracyclic Core of Nanolobatolide
Le Chang , Hao Jiang , Junkai Fu , Bin Liu , Chuang-chuang Li, and Zhen Yang
A concise synthetic pathway that enables the stereoselective construction of the tetracyclic core of nanolobatolide has been developed by applying a tandem ring-closing metathesis (RCM) reaction of dienynes, a Eu(fod)3-catalyzed intermolecular Diels–Alder reaction, and a biomimetic epoxide opening reaction as key steps.
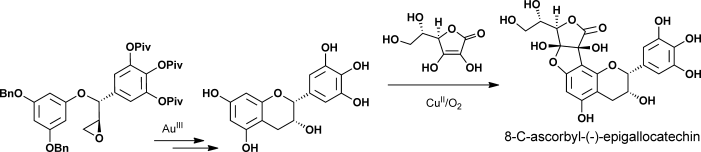
68.Enantioselective Total Syntheses of (+)-Gallocatechin, (−)-Epigallocatechin, and 8-C-Ascorbyl-(−)-epigallocatechin
Dr. Guang Lin, Dr. Le Chang, Dr. Yongxiang Liu, Dr. Zheng Xiang, Prof. Dr. Jiahua Chen,* andProf. Dr. Zhen Yang,*
Reading the tea leaves: The enatioselective total syntheses of 8-C-ascorbyl-(−)-epigallocatechin was accomplished by CuII-mediated oxidative coupling of ascorbic acid and (−)-epigallocatechin as a key step. Also, the asymmetric total syntheses of tea-leaf extracts (+)-gallocatechin and (−)-epigallocatechin were achieved by Au-catalyzed intramolecular cycliarylation of the precursor epoxide and Sharpless dihydroxylation.