Publications
142.Asymmetric Total Synthesis of (−)-Guignardones A and B
Zhiming Yan, Chunbo Zhao, Jianxian Gong,* and Zhen Yang*
Org. Lett. 2020, 22(4), 1644-1647
The asymmetric total synthesis of (−)-guignardones A (2) and B (1) has been accomplished. The highly oxidized 6-oxabicyclo[3.2.1]octane core was constructed from d-quinic acid via substitution/desulfurization reaction with thiophenol to forge the bridged ring scaffold, and a Pummerer rearrangement and 1,4-addition/elimination sequence was employed to install the β-carbonyl group at the congested C-1 position. A late-stage Knoevenagel condensation–6π-electrocyclization and directed hydrogenation formed (−)-guignardone B (1), which was subjected to dehydration to furnish (−)-guignardone A (2).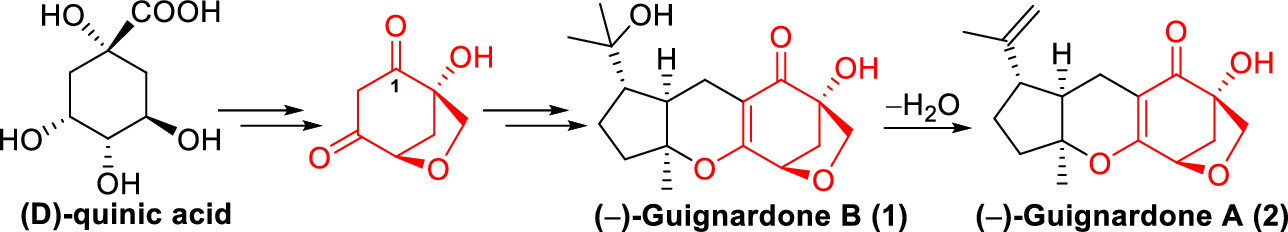
141.Protecting-Group-Free Total Syntheses of (±)-Norascyronones A and B
Tingting Cao, Lei Zhu, Yu Lan*, Jun Huang* and Zhen Yang*
Org. Lett. 2020, 22(7), 2517-2521
Protecting-group-free total syntheses of natural products norascyronone A and norascyronone B were accomplished in eight steps from the commercially available starting material 1-bromo-4-methoxy-2-methylbenzene. The key step was a Mn/Cu-mediated oxidative cascade annulation reaction that formed the tetracyclic core of the target molecules bearing vicinal bridge-head all-carbon quaternary chiral centers. Our investigation indicated that the C5 stereogenic center of norascyronone C plays a critical role in the proposed biomimetic oxidative reaction for B-ring formation.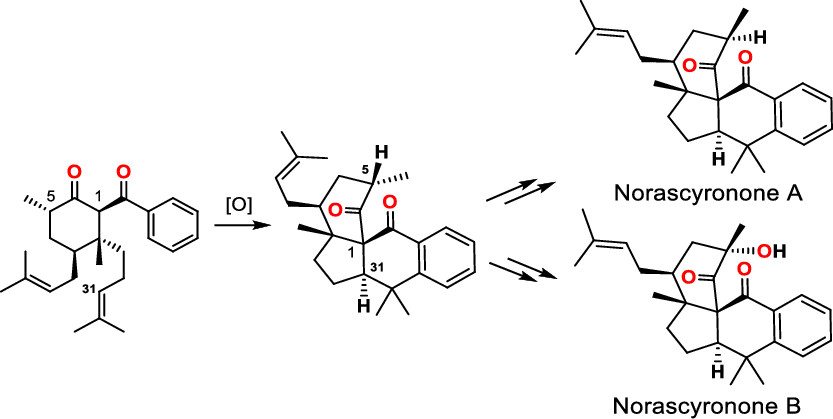
140.Retro-metal-ene versus retro-Aldol: mechanistic insight into Rh-catalysed formal [3+2] cycloaddition
Song Liu, Tao Zhang, Lei Zhu, Kangbao Zhong, Jianxian Gong, Zhen Yang*, Ruopeng Bai*, Yu Lan*
Chem. Commun., 2018,54(96), 13551-13554
Theoretical calculations have been performed to investigate the mechanism and stereoselectivity of rhodium-catalysed intramolecular [3+2] cycloaddition for construction of a substituted hexahydropentalene complex. A new C–C bond cleavage mechanism, retro-Aldol-type, is proposed and verified for this Rh-catalysed [3+2] cycloaddition reaction.
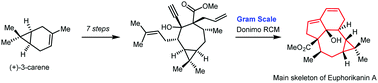
139.Concise gram-scale synthesis of Euphorikanin A skeleton through a domino ring-closing metathesis strategy
Linlin Shi, Yingdong He, Jianxian Gong *, Zhen Yang *
Chem. Commun. 2020,56(4), 531-534
Euphorikanin A is a diterpenoid possessing a highly congested and unprecedented 5/6/7/3-fused tetracyclic ring skeleton. To access the challenging chemical structure of Euphorikanins, an efficient total synthetic approach is described. The stereoselective synthesis of the core structure of Euphorikanin A has been achieved from a simple dienyne building block, and a domino ring-closing metathesis (RCM) strategy was used for the gram-scale synthesis of the highly strained Euphorikanin A core. This paves the way for the synthesis of structurally diverse Euphorikanins.
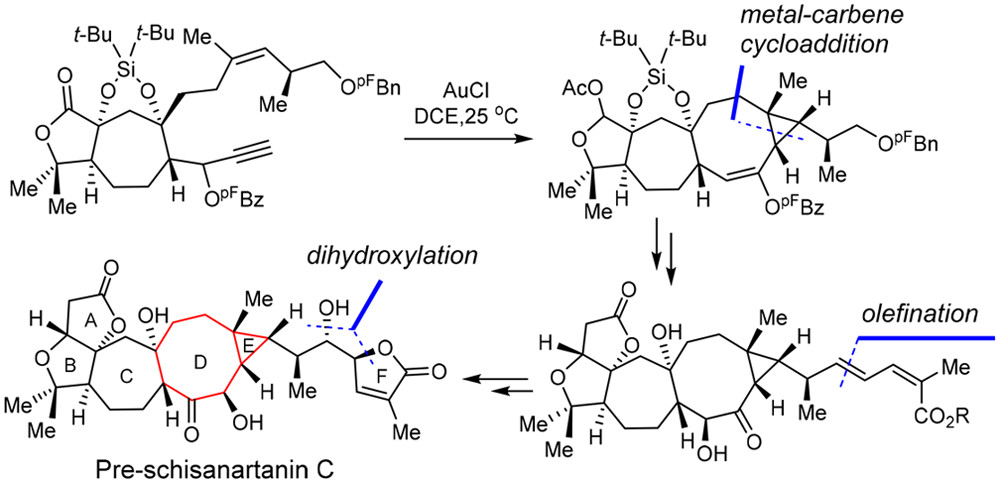
138.Asymmetric Total Synthesis of Pre-schisanartanin C
Yan-Long Jiang, Hai-Xin Yu, Yong Li, Pei Qu, Yi-Xin Han, Jia-Hua Chen*, Zhen Yang*
J. Am. Chem. Soc.2020, 142(1), 573-580
Pre-schisanartanin C belongs to the family of Schisandra nortriterpenoids with potent antihepatitis, antitumor, and anti-HIV activities. This paper presents the enantioselective total synthesis of pre-schisanartanin C (1). An important step in the total synthesis of 1 is gold-catalyzed intramolecular cyclopropanation of an 1,8-enyne substrates bearing a secondary ester group at the propargylic position to prepare a bicyclo[6.1.0]nonane core. Additional highlights include i) an asymmetric Diels–Alder reaction to install the initial C5 stereogenic center of 1, and ii) a sequential Pd-catalyzed Stille coupling, regio- and stereo-selective Sharpless asymmetric dihydroxylation, and a subsequent intramolecular lactonization to contruct the side chain of 1. The developed chemistry paves the way for the total syntheses of other family members bearing highly rigid bicyclo[6.1.0]nonane cores.