Publications
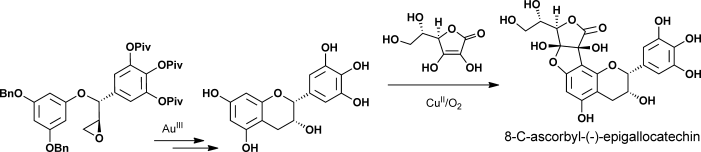
70.Enantioselective Total Syntheses of (+)-Gallocatechin, (−)-Epigallocatechin, and 8-C-Ascorbyl-(−)-epigallocatechin
Dr. Guang Lin, Dr. Le Chang, Dr. Yongxiang Liu, Dr. Zheng Xiang, Prof. Dr. Jiahua Chen,*, Prof. Dr. Zhen Yang*
Reading the tea leaves: The enatioselective total syntheses of 8-C-ascorbyl-(−)-epigallocatechin was accomplished by CuII-mediated oxidative coupling of ascorbic acid and (−)-epigallocatechin as a key step. Also, the asymmetric total syntheses of tea-leaf extracts (+)-gallocatechin and (−)-epigallocatechin were achieved by Au-catalyzed intramolecular cycliarylation of the precursor epoxide and Sharpless dihydroxylation.
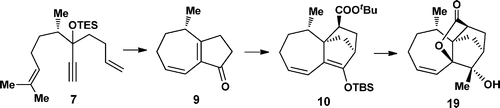
69.Synthesizing the Tetracyclic Core of Nanolobatolide
Le Chang , Hao Jiang , Junkai Fu , Bin Liu , Chuang-chuang Li, and Zhen Yang
A concise synthetic pathway that enables the stereoselective construction of the tetracyclic core of nanolobatolide has been developed by applying a tandem ring-closing metathesis (RCM) reaction of dienynes, a Eu(fod)3-catalyzed intermolecular Diels–Alder reaction, and a biomimetic epoxide opening reaction as key steps.
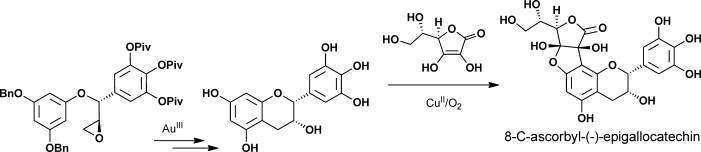
68.Enantioselective Total Syntheses of (+)-Gallocatechin, (−)-Epigallocatechin, and 8-C-Ascorbyl-(−)-epigallocatechin
Dr. Guang Lin, Dr. Le Chang, Dr. Yongxiang Liu, Dr. Zheng Xiang, Prof. Dr. Jiahua Chen,* andProf. Dr. Zhen Yang,*
Reading the tea leaves: The enatioselective total syntheses of 8-C-ascorbyl-(−)-epigallocatechin was accomplished by CuII-mediated oxidative coupling of ascorbic acid and (−)-epigallocatechin as a key step. Also, the asymmetric total syntheses of tea-leaf extracts (+)-gallocatechin and (−)-epigallocatechin were achieved by Au-catalyzed intramolecular cycliarylation of the precursor epoxide and Sharpless dihydroxylation.
67.Total Synthesis of (±)-Decinine via an Oxidative Biaryl Coupling with Defined Axial Chirality
Zhen-Hua Shan, Ji Liu, Ling-Min Xu, Ye-Feng Tang, Jia-Hua Chen, and Zhen Yang
The total synthesis of (±)-decinine has been achieved. The key steps in the synthesis involved the formation of lasubine II via a gold catalyzed annulation of 1-(but-3-yn-1-yl)piperidine and the formation of the 12-membered ring of decinine (1) with complementary atropselectivity via a VOF3-mediated oxidative biaryl coupling reaction.

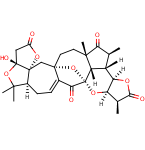
66.Diastereoselective Total Synthesis of (±)-Schindilactone A, Part 1: Construction of the ABC and FGH Ring Systems and Initial Attempts to Construct the CDEF Ring System
Tian-Wen Sun, Dr. Wei-Wu Ren, Dr. Qing Xiao, Prof. Dr. Ye-Feng Tang, Prof. Dr. Yan-Dong Zhang, Yong Li, Fan-Ke Meng, Yi-Fan Liu, Ming-Zhe Zhao, Ling-Min Xu, Prof. Dr. Jia-Hua Chen,*, Prof. Dr. Zhen Yang,*
First-generation synthetic strategies for the diastereoselective total synthesis of schindilactone A (1) are presented and methods for the synthesis of the ABC, FGH, and CDEF moieties are explored. We have established a method for the synthesis of the ABC moiety, which included both a Diels–Alder reaction and a ring-closing metathesis as the key steps. We have also developed a method for the synthesis of the FGH moiety, which involved the use of a Pauson–Khand reaction and a carbonylative annulation reaction as the key steps. Furthermore, we have achieved the construction of the central 7–8 bicyclic ring system by using a [3,3]-rearrangement as the key step. However, unfortunately, when this rearrangement reaction was applied to the construction of the more advanced CDEF moiety, the anticipated annulation reaction did not occur and the development of an alternative synthetic strategy would be required for the construction of this central core.