Publications
77. Asymmetric Total Synthesis of (+)-Fusarisetin A via the Intramolecular Pauson–Khand Reaction
Jun Huang, Lichao Fang, Rong Long, Li-Li Shi, Hong-Juan Shen, Chuang-chuang Li *, and Zhen Yang *
• Top10 Most Accessed Article in 07/2013.
• Highlighted in Synfacts, 2014, 10, 8.
An asymmetic total synthesis of (+)-fusarisetin A has been achieved. The essential to our strategy was the application of the intramolecular Pauson–Khand reaction for the stereoselective construction of the trans-decalin subunit of (+)-fusarisetin A with a unique C16 quarternary chiral center. The developed chemistry offers an alternative to the IMDA reaction that has been used for fusarisetin A, and is applicable to analogue synthesis for biological evaluation.

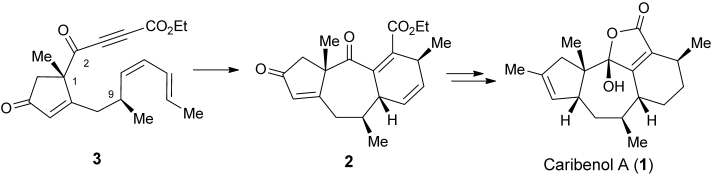
76.Asymmetric, Protecting-Group-Free Total Synthesis of (+)-Caribenol A
Jing-Chun Han, Dr. Lian-Zhu Liu, Prof. Dr. Chuang-Chuang Li,*, Prof. Dr. Zhen Yang,*
• Highlighted in Synfacts, 2013, 9(8)3, 809
Caribenol Queen: A new asymmetric, protecting-group-free synthesis of the marine tetracyclic diterpenoid (+)-caribenol A (1) has been achieved. The enantioselective synthesis employed (S)-methyl 1-methyl-2-oxocyclopent-3-enecarboxylate as a chiral scaffold, and an intramolecular Diels–Alder (IMDA) reaction of substrate 3 afforded the [5.7.6] tricyclic core in compound 2.
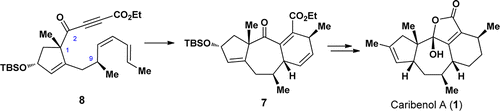
75.Asymmetric Total Synthesis of Caribenol A via an Intramolecular Diels−Alder Reaction
Jing-Chun Han, Lian-Zhu Liu, Yuan-Yuan Chang, Guo-Zong Yue, Jie Guo, Li-Yan Zhou, Chuang-Chuang Li*, and Zhen Yang*
-
• Highlighted in Synfacts, 2013, 9, 809.
• Highlighted in Org. Chem. Highlights, December, 2013.
A total synthesis of the caribenol A (1), a novel natural product with an intriguing tetracyclic framework, has been achieved. The synthesis features an intramolecular Diels–Alder (IMDA) reaction for the facile construction of the tricyclic [5–7–6] skeleton of caribenol A (1) and a biomimetic oxidation reaction for the formation of the 2-hydroxyfuran-2(5H)-one motif of caribenol A (1) as key steps. This synthetic approach also reveals that the sp2 carbon at C(2) in substrate 8 is a critical factor for the formation of the tricyclic [5–7–6] skeleton in 7.
74.Highly Regioselective Syntheses of Substituted Triphenylenes from 1,2,4-Trisubstituted Arenes via a Co-Catalyzed Intermolecular Alkyne Cyclotrimerization
Lingmin Xu, Ruocheng Yu, Yuefan Wang, Jiahua Chen, and Zhen Yang
J. Org. Chem., 2013, 78 , 5744
Herein, we report the development of a new method for the syntheses of substituted triphenylenes from the corresponding 1,2,4-trisubstituted arenes, which were themselves generated in a highly regioselective manner according to an intermolecular alkyne cyclotrimerization reaction that was catalyzed by a novel Co–TMTU complex. This highly regioselective reaction for the formation of 1,2,4-trisubstituted arenes will be a valuable addition to the plethora of tools already available to synthetic chemists and encourage further mechanistic studies of this important alkyne trimerization process.

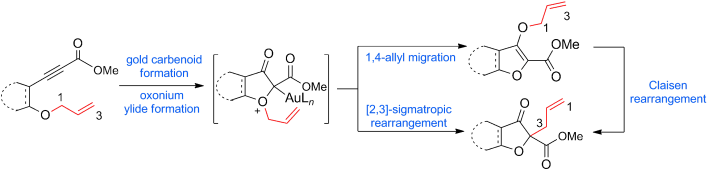
73.Gold-Catalyzed Rearrangement of Allylic Oxonium Ylides: Efficient Synthesis of Highly Functionalized Dihydrofuran-3-ones
Junkai Fu, Dr. Hai Shang, Zhaofeng Wang, Dr. Le Chang, Wenbing Shao, Prof. Dr. Zhen Yang, Prof. Dr. Yefeng Tang
Angew. Chem. Int. Ed. 2013, 52, 4198
“Diazo” not needed: The title reaction results in the rearrangement of oxonium ylides, which were prepared from readily available homopropargylic allylic ethers instead of diazo compounds, through two different mechanisms: a concerted 2,3-sigmatropic rearrangement, or a stepwise 1,4-allyl migration followed by a Claisen rearrangement (see scheme).