Publications
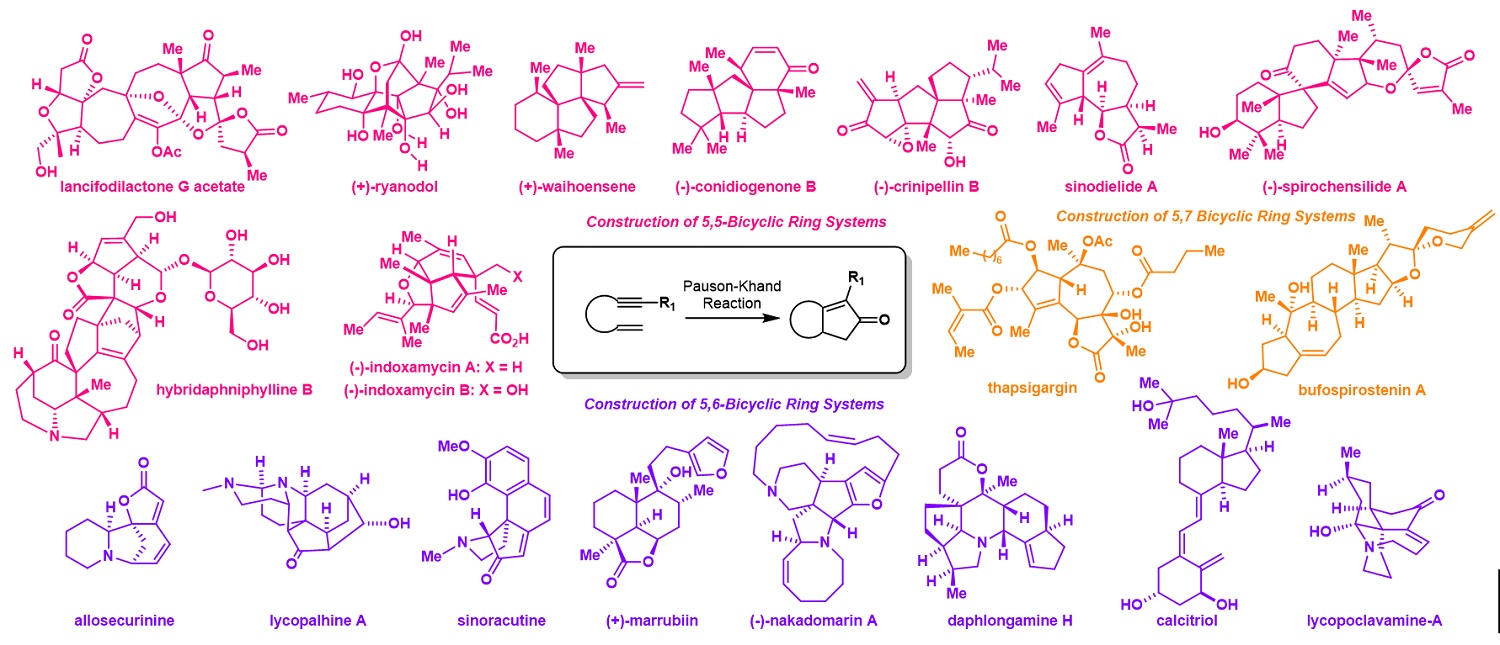
147.Evolution of Pauson-Khand Reaction: Strategic Applications in Total Syntheses of Architecturally Complex Natural Products (2016–2020)
Sijia Chen†, Chongguo Jiang†, Nan Zheng, Zhen Yang* and Lili Shi*
Catalysts, 2020, 10 (10), 1199
Metal-mediated cyclizations are important transformations in a natural product total synthesis. The Pauson-Khand reaction, particularly powerful for establishing cyclopentenone-containing structures, is distinguished as one of the most attractive annulation processes routinely employed in synthesis campaigns. This review covers Co, Rh, and Pd catalyzed Pauson-Khand reaction and summarizes its strategic applications in total syntheses of structurally complex natural products in the last five years. Additionally, the hetero-Pauson-Khand reaction in the synthesis of heterocycles will also be discussed. Focusing on the panorama of organic synthesis, this review highlights the strategically developed Pauson-Khand reaction in fulfilling total synthetic tasks and its synthetic attractiveness is aimed to be illustrated.
146.Synthesis of 4-Desmethyl-Rippertenol and 7-Epi-Rippertenol via Photoinduced Cyclization of Dienones
Zi-Chun Zhang†, Dan-Dan Zhao†, Zhong-Chao Zhang, Xin-Yu Tan, Jian-Xian Gong*, Jun-Kai Fu* & Zhen Yang*
Chinese Chemical Society, 2020, 2(5), 2074–2083
The synthesis of cycloheptanoid-based fused polycyclic frameworks is a challenge for organic chemists due to unfavorable entropic factors and ring strains. Herein, a concise synthesis of 4-desmethyl-rippertenol and 7-epi-rippertenol bearing a unique, [6,6,5,7]-fused tetracyclic framework is reported. The route features a novel photoinduced intramolecular cyclization of α-cyclopropyl dienone followed by an unexpected thermal 1,5-hydrogen migration, which provides efficient access to the fused seven-membered ring system in a stereochemically well-defined manner. Further density functional theory (DFT) calculations disclose that the stereoselectivity of this photoinduced process is mainly attributed to transition state conformation and steric effects.
145.Asymmetric Total Synthesis of (−)-Spirochensilide A
Xin-Ting Liang, Jia-Hua Chen,* and Zhen Yang*
J. Am. Chem. Soc. 2020, 142(18), 8116–8121
An asymmetric total synthesis of (−)-spirochensilide A has been achieved for the first time. The synthesis features a semipinacol rearrangement reaction to stereoselectively construct the two-vicinal quaternary chiral centers at C8 and C10, a tungsten-mediated cyclopropene-based Pauson–Khand reaction to install the C13 quaternary chiral center, and a furan-based oxidative cyclization to stereoselectively form the spiroketal motif.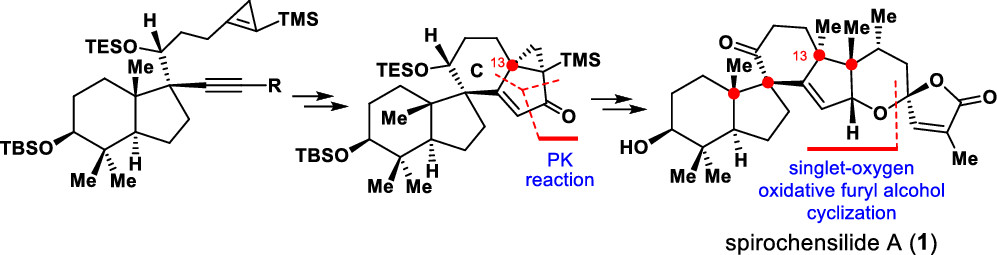
144.Photoredox‐Catalyzed Isomerization of Highly‐Substituted Allylic Alcohols via C‐H bond activation
Kai Guo, Zhongchao Zhang, Anding Li, Yuanhe Li, Jun Huang,* Zhen Yang,*
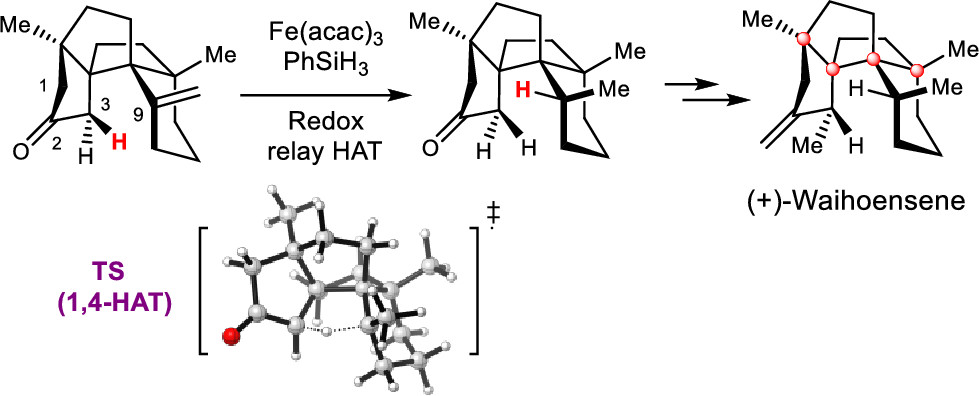
143.Asymmetric Total Synthesis of (+)-Waihoensene
Yongzheng Qu, Zheyuan Wang, Zhongchao Zhang, Wendou Zhang, Jun Huang,* and Zhen Yang*
J. Am. Chem. Soc. 2020, 142(14), 6511-6515
• Highlighted in ChemistryViews, 07 Apr 2020
The asymmetric total synthesis of (+)-waihoensene, which has a cis-fused [6,5,5,5] tetracyclic core bearing an angular triquinane, a cis-fused six-membered ring, and four contiguous quaternary carbon atoms, was achieved through a sequence of chemical reactions in a stereochemically well-defined manner. The total synthesis features the following: (1) Cu-catalyzed asymmetric conjugated 1,4-addition; (2) diastereoselective Conia-ene type reaction; (3) diastereoselective intramolecular Pauson–Khand reaction; (4) Ni-catalyzed diastereoselective conjugated 1,4-addition; and (5) radical-initiated intramolecular hydrogen atom transfer (HAT). Control experiments and density functional theory calculations support the proposed HAT process.