Publications
67.Total Synthesis of (±)-Decinine via an Oxidative Biaryl Coupling with Defined Axial Chirality
Zhen-Hua Shan, Ji Liu, Ling-Min Xu, Ye-Feng Tang, Jia-Hua Chen, and Zhen Yang
The total synthesis of (±)-decinine has been achieved. The key steps in the synthesis involved the formation of lasubine II via a gold catalyzed annulation of 1-(but-3-yn-1-yl)piperidine and the formation of the 12-membered ring of decinine (1) with complementary atropselectivity via a VOF3-mediated oxidative biaryl coupling reaction.

66.Diastereoselective Total Synthesis of (±)-Schindilactone A, Part 1: Construction of the ABC and FGH Ring Systems and Initial Attempts to Construct the CDEF Ring System
Tian-Wen Sun, Dr. Wei-Wu Ren, Dr. Qing Xiao, Prof. Dr. Ye-Feng Tang, Prof. Dr. Yan-Dong Zhang, Yong Li, Fan-Ke Meng, Yi-Fan Liu, Ming-Zhe Zhao, Ling-Min Xu, Prof. Dr. Jia-Hua Chen,*, Prof. Dr. Zhen Yang,*
First-generation synthetic strategies for the diastereoselective total synthesis of schindilactone A (1) are presented and methods for the synthesis of the ABC, FGH, and CDEF moieties are explored. We have established a method for the synthesis of the ABC moiety, which included both a Diels–Alder reaction and a ring-closing metathesis as the key steps. We have also developed a method for the synthesis of the FGH moiety, which involved the use of a Pauson–Khand reaction and a carbonylative annulation reaction as the key steps. Furthermore, we have achieved the construction of the central 7–8 bicyclic ring system by using a [3,3]-rearrangement as the key step. However, unfortunately, when this rearrangement reaction was applied to the construction of the more advanced CDEF moiety, the anticipated annulation reaction did not occur and the development of an alternative synthetic strategy would be required for the construction of this central core.
65.Diastereoselective Total Synthesis of (±)-Schindilactone A, Part 2: Construction of the Fully Functionalized CDEFGH Ring System
Yong Li, Zhi-Xing Chen, Dr. Qing Xiao, Qin-Da Ye, Tian-Wen Sun, Fan-Ke Meng, Dr. Wei-Wu Ren, Lin You, Ling-Min Xu, Yue-Fan Wang, Dr. Jia-Hua Chen,*, Dr. Zhen Yang,*
The successful synthesis of the highly complex model compound (2) of the CEFGH ring system of schindilactone A (1) is described. Several synthetic methodologies were developed and applied to achieve this goal, including ring-closing metathesis (RCM) and Co–thiourea-catalyzed Pauson–Khand reactions. Furthermore, two independent approaches were developed for the construction of the GH ring of model compound 2, the key steps of which included Pd–thiourea-catalyzed carbonylative annulation, methylation, and sequential RCM/oxa-Michael-addition reactions. The chemistry developed herein has provided a greater understanding of the synthesis of schindilactone A (1) and its analogous compounds of the same family.
64.Diastereoselective Total Synthesis of (±)-Schindilactone A, Part 3: The Final Phase and Completion
Dr. Wei-Wu Ren, Zhi-Xing Chen, Dr. Qing Xiao, Yong Li, Tian-Wen Sun, Zi-Yang Zhang, Qin-Da Ye, Fan-Ke Meng, Lin You, Ming-Zhe Zhao, Ling-Min Xu, Prof. Dr. Ye-Feng Tang,*, Prof. Dr. Jia-Hua Chen,*, Prof. Dr. Zhen Yang,*
The final phase for the total synthesis of (±)-schindilactone A (1) is described herein. Two independent synthetic approaches were developed that featured Pd–thiourea-catalyzed cascade carbonylative annulation reactions to construct intermediate 3 and a RCM reaction to make intermediate 4. Other important steps that enabled the completion of the synthesis included: 1) A Ag-mediated ring-expansion reaction to form vinyl bromide 17 from dibromocyclopropane 30; 2) a Pd-catalyzed coupling reaction of vinyl bromide 17 with a copper enolate to synthesize ketoester 16; 3) a RCM reaction to generate oxabicyclononenol 10 from diene 11; 4) a cyclopentenone fragment in substrate 8 was constructed through a Co–thiourea-catalyzed Pauson–Khand reaction (PKR); 5) a Dieckmann-type condensation to successfully form the A ring of schindilactone A (1). The chemistry developed for the total synthesis of schindilactone A (1) will shed light on the synthesis of other family members of schindilactone A.

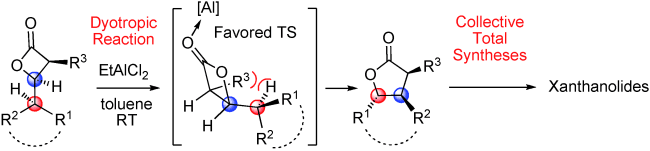
63.Enantioselective and Collective Syntheses of Xanthanolides Involving a Controllable Dyotropic Rearrangement of cis-b -Lactones
W. W. Ren, Y. C. Bian, Z. Y. Zhang, H. Shang, P. T. Zhang, Y. J. Chen, Z. Yang*, T. P. Luo*, and Y. F. Tang*
Angew. Chem. Int. Ed. 2012, 51, 6984
Let’s swap: A scalable, atom-economic, enantio-, and diastereoselective synthetic route to trisubstituted γ-butyrolactones based on a Wagner–Meerwein-type dyotropic rearrangement of cis-β-lactones is described (see scheme). This methodology was applied in efficient and protecting-group-free formal syntheses and total syntheses of various xanthanolide natural products.